Buckyballs and Nanotubes: Carbon's Amazing Shapes
Carbon - The Amazing Shapeshifter!
Buckyballs and Nanotubes are special forms of the element Carbon. Carbon is one of the most abundant elements and is the basis of life. Like they say in sci-fi shows, we are "carbon-based" life forms.
Carbon is an amazingly flexible element and can take on many forms, or allotropes.
Carbon's Amazing Forms (Allotropes)
The same carbon atoms can arrange themselves in completely different ways, creating materials with totally different properties!
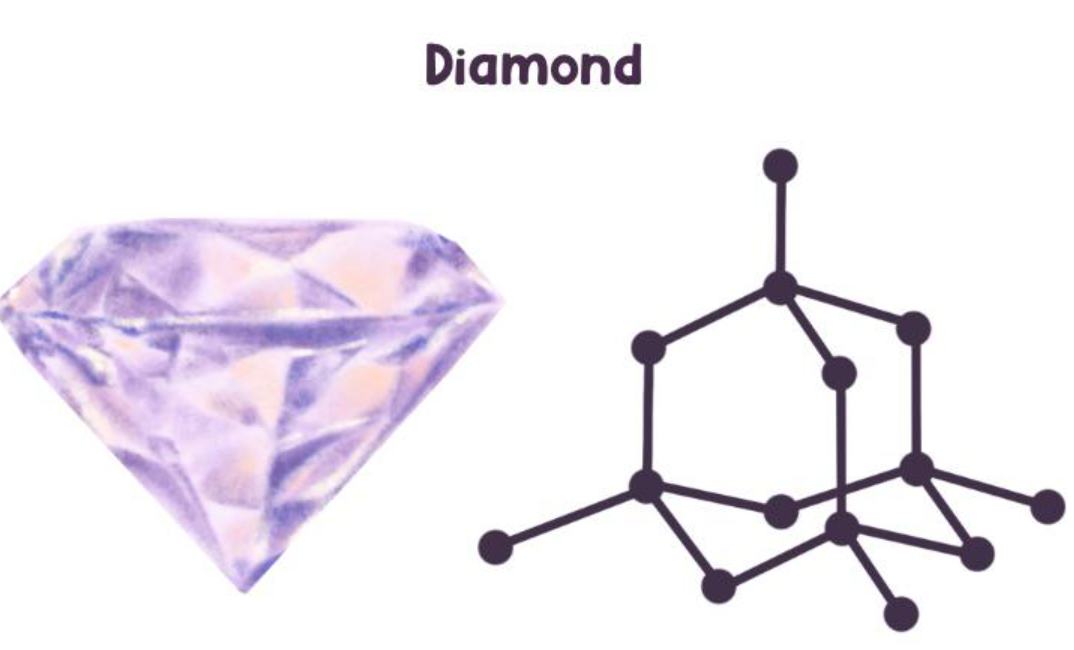
Diamond
Structure: 3D tetrahedral network
Properties: Hardest natural material, excellent thermal conductor, does not conduct electricity
Uses: Jewelry, cutting tools, drill bits
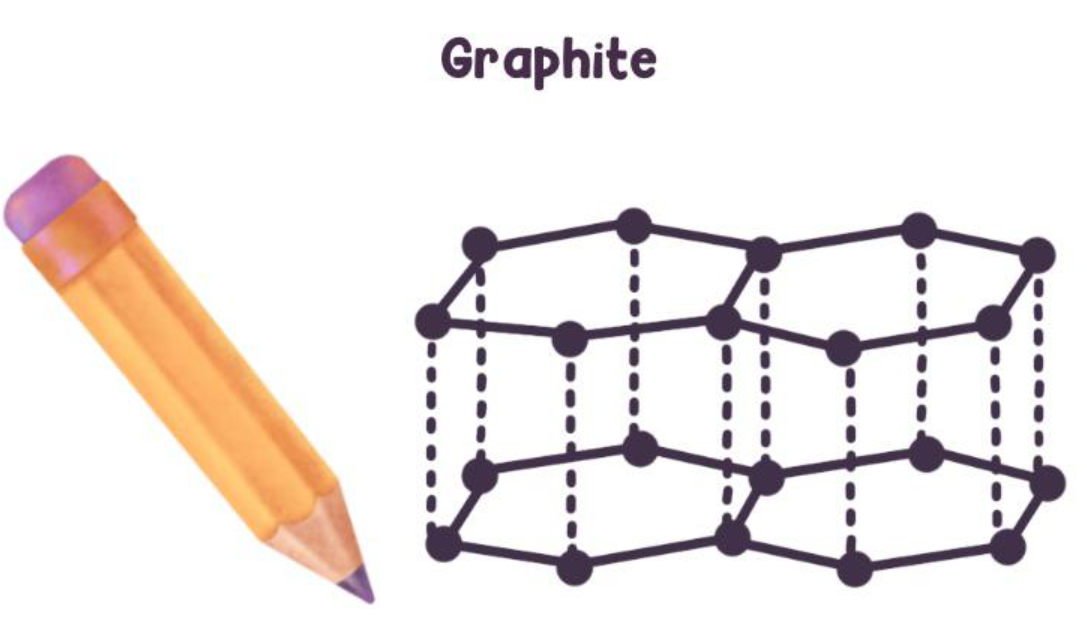
Graphite
Structure: Layered hexagonal sheets
Properties: Soft, slippery, conducts electricity
Uses: Pencil "lead," lubricants, electrodes

Graphene
Structure: Single layer of hexagons
Properties: Strongest 2D material, excellent conductor
Uses: Future electronics, sensors, composites

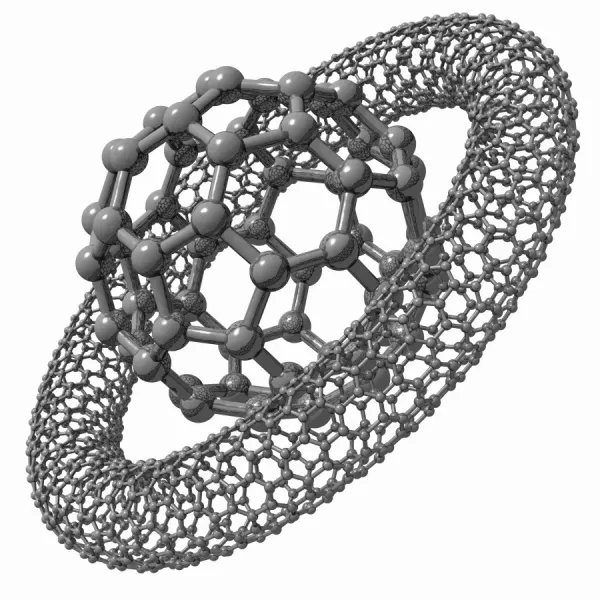
Buckyballs (C60)
Structure: Hollow sphere (soccer ball shape)
Properties: Unique chemistry, can trap other atoms inside
Uses: Drug delivery, lubricants, superconductors

Carbon Nanotubes
Structure: Rolled graphene sheets forming tubes
Properties: Incredibly strong, can conduct or insulate
Uses: Composites, electronics, sensors
Amazing Fact:
All of these different materials are made from exactly the same type of atom - carbon! The only difference is how the atoms are arranged and connected to each other. This shows how important molecular structure is in determining a material's properties.
Focus on Buckyballs
Imagine a molecule that looks exactly like a soccer ball - that's a buckyball! These amazing carbon molecules, officially called Buckminsterfullerene (or C60), are made of 60 carbon atoms arranged in a perfect hollow sphere.
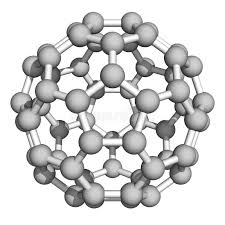
They were so revolutionary that the scientists who discovered them won the Nobel Prize!
Buckyballs got their name from architect Buckminster Fuller, who designed geodesic domes with similar patterns.
Understanding the Nano Scale
Buckyballs and nanotubes are really small! To understand how tiny they are, let's use some comparisons:
- 1 nanometer: Size of a buckyball
- 10 nanometers: Width of DNA molecule
- 100 nanometers: Size of a virus
- 1,000 nanometers (1 micrometer): Size of a bacteria
- 10,000 nanometers: Thickness of spider silk
- 100,000 nanometers: Width of human hair
Size Check!
Buckyballs are so tiny that millions could fit on the period at the end of this sentence. If you enlarged a buckyball to the size of a soccer ball, and then enlarged an actual soccer ball by the same amount, the soccer ball would be as big as the Earth!
Buckyball Quick Facts
- Size: About 1 nanometer in diameter (1 billionth of a meter)
- Structure: 60 carbon atoms in 12 pentagon and 20 hexagon patterns
- Discovery: 1985 by Harold Kroto, Robert Curl, and Richard Smalley
- Nobel Prize: 1996 Chemistry Prize for their discovery
- Found in space: Detected around stars and in cosmic dust
Focus on Carbon Nanotubes
A carbon nanotube tube is made of a
sheet of graphene that's been rolled up. (Like
a toilet paper tube). Sometimes, other tubes
are wrapped around the first tube in concentric circles.
These tubes are incredibly
strong and have amazing properties.
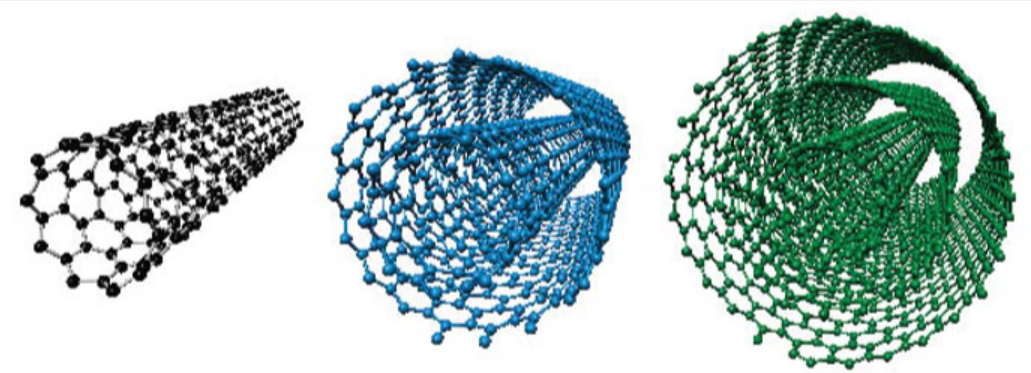
Properties of Nanotubes
- 100 times stronger than steel but 1/6th the weight. A nanotube cable just 1mm thick could lift a car
- Better at conducting electricity than copper. In fact, a single nanotube can conduct electricity, act as a transistor, or emit light - it's like having a complete electronic device in one molecule!
- Can be twisted and bent without breaking. If you could unroll all the nanotubes in a pencil-sized piece of nanotube material, they would stretch around Earth's equator
- Nanotubes can be used to build incredibly sensitive detectors that can detect single particles!
Building the Future
Scientists are using buckyballs and nanotubes to create incredible new technologies:
- Super Sensors: Used to detect single atoms and molecules
- Space Elevators: Ultra-strong cables that could reach into space
- Tiny Computers: Microscopic electronic circuits
Buckyballs in Thunder Cloud Summer
In our story, Professor Shug explains how his detector uses buckyballs and nanotubes:
- Special atoms inside tubes: Scientists really do put other atoms inside nanotubes to create new materials
- Particle detection: The unique properties of nanotubes make them excellent for sensing cosmic rays
- Expensive to make: The professor mentions the high cost - real nanotube production is indeed very expensive
- Spiral structure: The detector's black spirals could represent coiled nanotubes
While Professor Shug's detector is fictional, scientists really are using carbon nanotubes in sensors, electronics, and even in research to detect particles from space!
Try it Yourself!
1. Create a Buckyball Model
Materials:
- Sheets of card stock paper
- Scissors
- Pencils
- Tape
Instructions:
1. Cut 12 pentagons.
Here is a template with 3 different sizes of pentagons to choose from.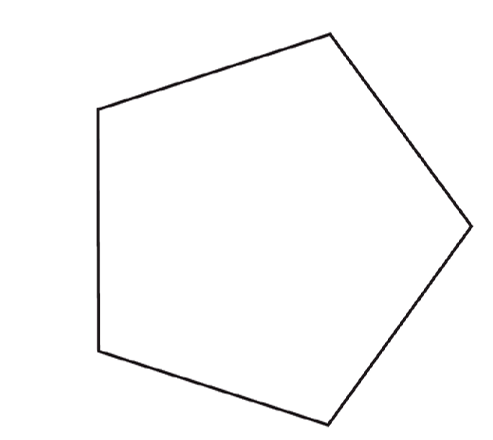
2. Cut 20 hexagons. Make sure to use a hexagon shape with the same side length as the pentagon from Step 1.
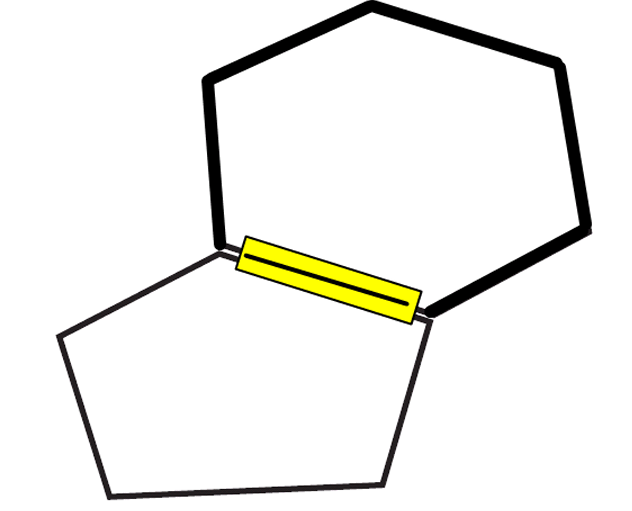
3. Tape a hexagon side to a pentagon side
4. Tape 4 more hexagons to the pentagon. You will have to lift the hexagons up slightly as you tape. (The surface should curve up)
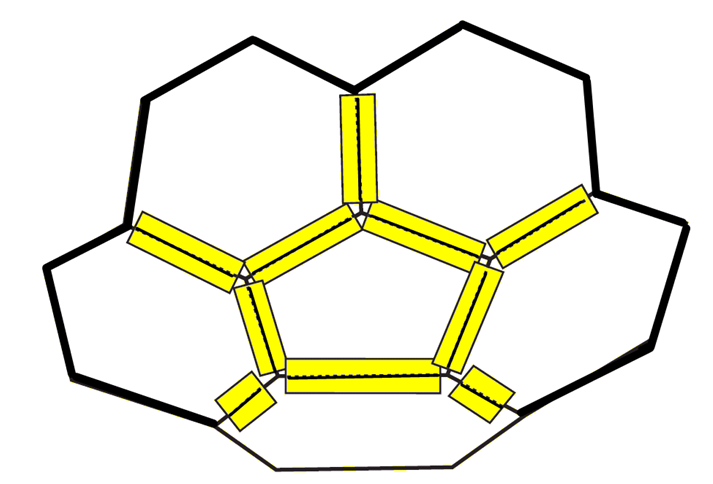
5. Tape 5 pentagons at the places where the hexagon edges come together, as shown. Again, you will have to lift the pentagons up slightly as you tape.
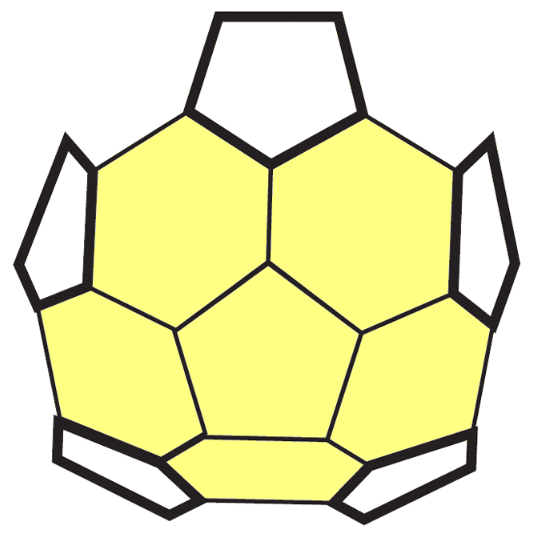
6. Fill in the "gaps" in the surface with 5 more hexagons, as shown. This completes half of the shape, using 10 hexagons and 6 pentagons.
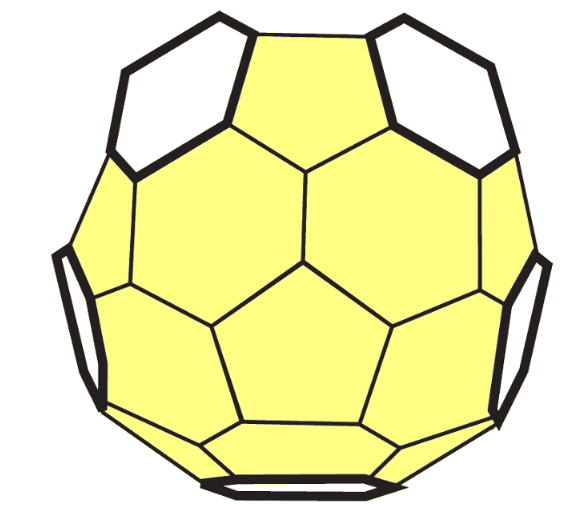
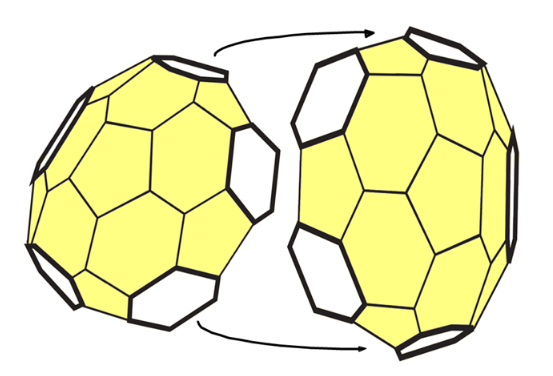
7. Repeat steps 3-6 to build the other half. Then, tape the two halves together. You've made a Buckyball!
Why This Pattern?
The pentagon-hexagon pattern is the only way to arrange exactly 60 carbon atoms into a stable hollow sphere. It's the same pattern soccer ball makers use because it creates the most perfect sphere possible!
2. Nanotube Strength Test
Carbon nanotubes are incredibly strong, due to their geometric shape as a cylinder. In this activity, we will compare the strength of a flat surface (like graphene) to that of a cylinder.
Materials:
- Two tables, chairs, or desks of equal height
- 12 pieces of paper
- Small weights - all of the same type (e.g. coins, washers)
- Scotch tape or rubber bands
- Plastic cup
- Sturdy string or thin ribbon
- Hole punch or sharp knife (adult supervision required for knife)
Instructions:
- Set up your two identical tables, chairs, or desks so they are next to each other, with a two to three inch gap in between.
- Stack six sheets of paper on top of each other, so they bridge the gap between your two tables. Center the sheets across the middle of the gap.
- Use the hole punch or knife (adult supervision required for knife) to punch two holes on opposite sides of the cup, near the rim at the top. Cut a piece of string several feet long, and tie one end of the string securely to each hole.
- Hang the plastic cup from the sheets of paper, so the string goes directly across the middle of the sheets (centered in the gap between the tables).
- One by one, add weights to the plastic cup until the paper falls (note: if the string breaks before the paper, re-tie the knot or use a thicker string, then restart the experiment). How many pennies does it take to make the paper fall? What happens to the paper? Does it bend gradually, or does it fold sharply in the middle?
- Now, roll six of your pieces of paper into tubes about one inch in diameter. Use Scotch tape or rubber bands to hold the paper in tube shapes.
- lay your six tubes of paper next to each other, with each one crossing the gap between the tables. Center the tubes across the middle of the gap. Make sure the tubes are pressed up against each other, with no space in between.
- Empty the plastic cup and hang it from the tubes, so the string goes around all six tubes, and is centered in the gap between the tables.
- One by one, add weights to the plastic cup until the tubes fall.
Extra Repeat the activity with different diameter paper tubes, for example half-inch diameter and two inch diameter tubes. As tubes get bigger (or smaller) can they hold more or less weight?
Extra Make a 7th tube and bundle 6 tubes around this new tube with tape (like multiple nanotubes). Test the bundle - how much more weight can it hold?
Just like how bundling tubes makes them stronger, scientists bundle carbon nanotubes to create super-strong materials. The tubes support each other and distribute stress more evenly!
Want to Learn More?
- National Nanotechnology Initiative - What is nanotechnology?
- Explain That Stuff - Nanotechnology
- Understanding Nano - Educational nanotechnology resources
- Nobel Prize Chemistry 1996 - The discovery of buckyballs
- NISE Network - Nanoscale science education resources